Zinc in the Environment
Introduction
Zinc is an essential mineral of “exceptional biologic and public health importance” and is considered a “Life Saving Commodity” by the United Nations. [1], [2]
Due to its unique properties, zinc is used in a wide range of consumer, infrastructure, agricultural, and industrial products. More importantly, zinc is essential to life, playing an important role in biological processes of all living organisms (humans, animals, and plants). Zinc is crucial for cell division, protein synthesis, the immune system and growth.
Natural Occurrence
Zinc is the 24th most abundant element in the Earth’s crust and has been present ever since the planet formed its surface. All life on earth has developed in the presence of zinc.
The concentration of zinc in nature without the additional influence of human activities (anthropogenic emissions) is called “natural background.” The natural background levels in surface water, soil and rock vary over a wide range of concentrations. Background levels of zinc in soil and rock typically range between 10 and 300 milligrams per kilogram, and zinc in rivers varies from less than 10 micrograms per liter to over 200 micrograms.
Zinc Cycling Through Nature
By natural erosion processes, a small part of the zinc in soil, rock and sediment is constantly moved and transported through the environment. Rain, snow, ice, solar heat and wind erode zinc-containing rocks and soil. Wind and water carry minute amounts of zinc to lakes, rivers and the sea, where it collects as sediment or is transported further. Natural phenomena such as volcanic eruptions, forest fires, dust storms and sea spray also contribute to the continuous cycling of zinc through nature. It is estimated that these natural emissions of zinc amount to 5.9 million metric tonnes each year. [3]
Human activities do not add to the overall zinc amount on a global scale. But mining, production of goods and the use of zinc create situations where emissions to the atmosphere, soil and water can occur. These are known as anthropogenic emissions, which are estimated to be a fraction of the total emissions from the natural cycling of zinc from erosion, sea spray, volcanic eruptions etc. [4]
Potential sources of anthropogenic zinc emissions include: the production and processing of zinc into products; emissions from power plants and other municipal and industrial sources not related to the zinc industry and; certain zinc applications where corrosion or abrasion may result in small releases of zinc to the environment, although these are generally widely dispersive in nature.
On a global scale, the influence of natural zinc cycling processes on environmental zinc levels is much more important than the influence from human activity. However, at a local scale, anthropogenic emissions can in some places outweigh natural processes.
Environmental Fate of Zinc
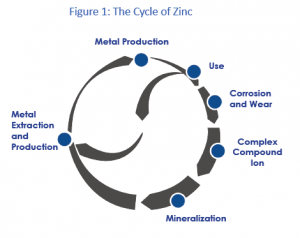 Zinc released to the environment follows a cycle (figure 1) in which zinc from mineral ore bodies is converted through extraction and refining processes from its mineral state (mostly sphalerite ore, zinc sulfide) into the metallic state.[5] Most of this metal will have a long service in stable metal applications and will be recovered and recycled at the end of life. Metallic zinc that is exposed to the atmosphere may be subject to corrosion that will result in a slow release of small amounts of zinc into the environment.
Zinc released to the environment follows a cycle (figure 1) in which zinc from mineral ore bodies is converted through extraction and refining processes from its mineral state (mostly sphalerite ore, zinc sulfide) into the metallic state.[5] Most of this metal will have a long service in stable metal applications and will be recovered and recycled at the end of life. Metallic zinc that is exposed to the atmosphere may be subject to corrosion that will result in a slow release of small amounts of zinc into the environment.
Zinc metal is also transformed into zinc compounds (e.g. zinc oxide, zinc chloride, zinc phosphate) that are used in a wide variety of applications. These uses may also result in small diffusive releases.
During the production and use phase of zinc, zinc compounds with varying solubility may be formed and be released into the environment. In addition to these emissions related to human activity, a natural flow of zinc will always cycle through the environment due to the natural processes of weathering and erosion. All these processes mobilize a variety of zinc compounds into the environment.
Once mobilized, zinc interacts with the different components of water, sediments and soil and ultimately partitions between different fractions in these environmental compartments. This interaction and the dynamic processes involved ultimately define zinc’s environmental fate, i.e. the form(s) in which the metal will be present in the environment and in which it will ultimately end up. In this respect, most of the zinc will return to the stable chemical form, often ZnS, from which it was originally mined. This “mineralization” back into stable chemical forms closes the “cycle.”
The original and ultimate chemical forms of zinc (mainly ZnS) are very stable, and the contained zinc has very low solubility and very low potential for uptake by organisms (bioavailability).
Environmental Effects of Zinc
The environmental impact of zinc – and of all essential elements – cannot be assessed in the same way as man-made chemical compounds. Because zinc occurs naturally, eliminating it from the environment would not be possible. Moreover, because zinc is essential, achieving such a goal would ultimately lead to 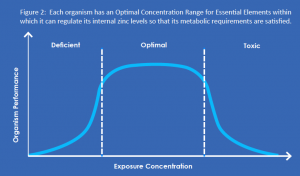 detrimental effects throughout an ecosystem. In other words, ‘less’ is not necessarily ‘better’.
detrimental effects throughout an ecosystem. In other words, ‘less’ is not necessarily ‘better’.
For essential elements such as zinc, environmental effects must be considered within the context of an organism’s natural ability to regulate (uptake and excretion) and maintain a certain level of homeostasis. That is, environments containing zinc at very low, or very high, concentrations may produce undesirable effects. The range between the minimum and maximum is often called the optimal window of essentiality (Figure 2). Organisms have evolved mechanisms to supply their needs independent of the external concentration by regulating an essential element to a constant internal level. [6]
Zinc Bioavailability
The characterization of risk for metals has evolved significantly over the past several decades and currently incorporates concepts of bioavailability. The term bioavailability refers to the form (species) of a metal that is able to enter an organism and elicit an effect. For zinc, the species typically considered to be the source of toxicity (bioavailable) is the uncomplexed, free ion (Zn2+). However, because zinc interacts with various constituents of water, soil and sediment, it can exist as many different complexes. (Figure 3)
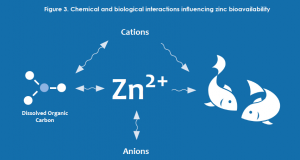 In water, zinc concentrations have traditionally been considered on the basis of the total (entire zinc pool in a sample) or dissolved (complexes that can pass through a 0.45 micrometer filter) fraction. However, even following filtration at microscopic levels, the dissolved fraction contains many zinc complexes other than the free ion.
In water, zinc concentrations have traditionally been considered on the basis of the total (entire zinc pool in a sample) or dissolved (complexes that can pass through a 0.45 micrometer filter) fraction. However, even following filtration at microscopic levels, the dissolved fraction contains many zinc complexes other than the free ion.
For example, increases in pH, alkalinity or natural organic matter would all tend to decrease zinc bioavailability through complexation. Similarly, zinc bioavailability may also be affected through competition with other positively charged ions (calcium, magnesium, sodium, etc.).
Although accounting for bioavailability in sediment and soils follows a conceptually similar framework as water, additional constituents must be considered. For sediments, zinc has the potential for complexation with iron and manganese oxides (minerals) or organic matter, or in the case of anaerobic sediment, with sulfides.[7] For soils, zinc is strongly adsorbed to mineral phases (oxides, silica, carbonate, clay particles) and organic matter, and the sorption tends to increase with increasing pH.[8] As a result, zinc in sediment and soil is available to complex with these additional compounds/surfaces, thereby decreasing its bioavailable form and potential toxicity to organisms.
In summary, zinc’s bioavailability is determined by complex interactions with the environment and is strongly dependent on the characteristics of that environment. In order to understand the many interactions that occur between zinc and the media in which it resides, computational tools have been developed to efficiently characterize site-specific scenarios. As a result, environmental protection levels for zinc typically are no longer expressed as a single value; instead they fluctuate with the ameliorative capacity of the media of interest.
Conclusions
The environmental assessment of metals requires a science-based approach because of the natural occurrence of metals, the great variations in metal speciation affecting the metal’s bioavailability and toxicity and – for metals such as zinc – their essentiality for all living organisms.
The distribution, transport and effects (bioavailability) of zinc in water, sediment and soil depend largely on the site-specific chemical and physical characteristics of the environment and an organism’s condition e.g. age, size, prior history of exposure. Thus environmental assessment of zinc must take these factors into account to be meaningful.
Studies using the state-of-the-science approach have concluded that current zinc uses contribute negligible amounts of bioavailable zinc to the environment and therefore have low potential for environmental effects.

